A clinical trial is underway to develop a coronavirus vaccine
The first participant in a clinical trial for a vaccine against COVID-19 will get an experimental dose today.
The trial for the vaccine, which is funded by the National Institutes of Health, is taking place at Seattle’s Kaiser Permanente Washington Health Research Institute. A government official spoke to the Associated Press about the trial anonymously because the agency hasn’t yet made an official public statement about it.
For the clinical trial, 45 young, healthy volunteers will receive different doses of the shots, which were co-developed by NIH and Moderna, Inc. The official told the AP that the participants in the clinical trial aren’t at risk of getting COVID-19 from the experimental dose because it doesn’t contain the coronavirus itself.
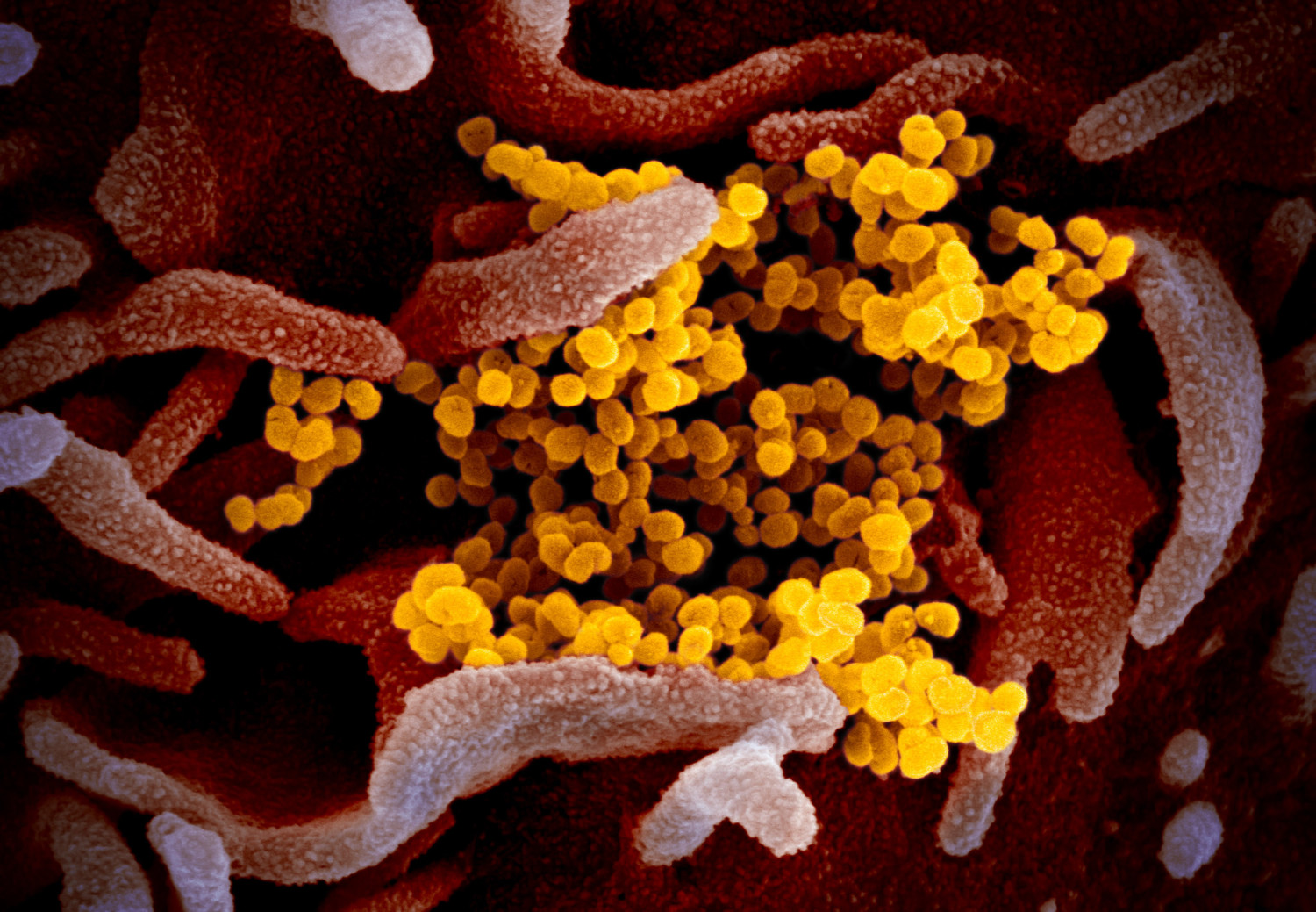
The vaccine relies on genetic engineering to prod our cells into producing a tiny piece of the virus, which will prompt our immune systems to attack it. In the experimental vaccine, a snippet of messenger RNA contains “instructions to build the receptor on the virus’ surface that allows the immune system to target it,” according to the Guardian. If it works, our cells will fight the receptor on the surface of the virus without us ever getting sick.
The trial is designed to ensure that the experimental vaccine doesn’t cause side effects. If all goes well, this trial will let researchers move forward with larger tests.
The New York Times reported that Moderna delivered the experimental vaccine to NIH in late February for early testing.
Research groups all over the world have been working to develop a vaccine for the new coronavirus. But this doesn’t mean you’ll be able to get a vaccine for COVID-19 soon. Public health officials have warned that it will take 18 months to develop and fully validate any vaccine that would be available to the public.