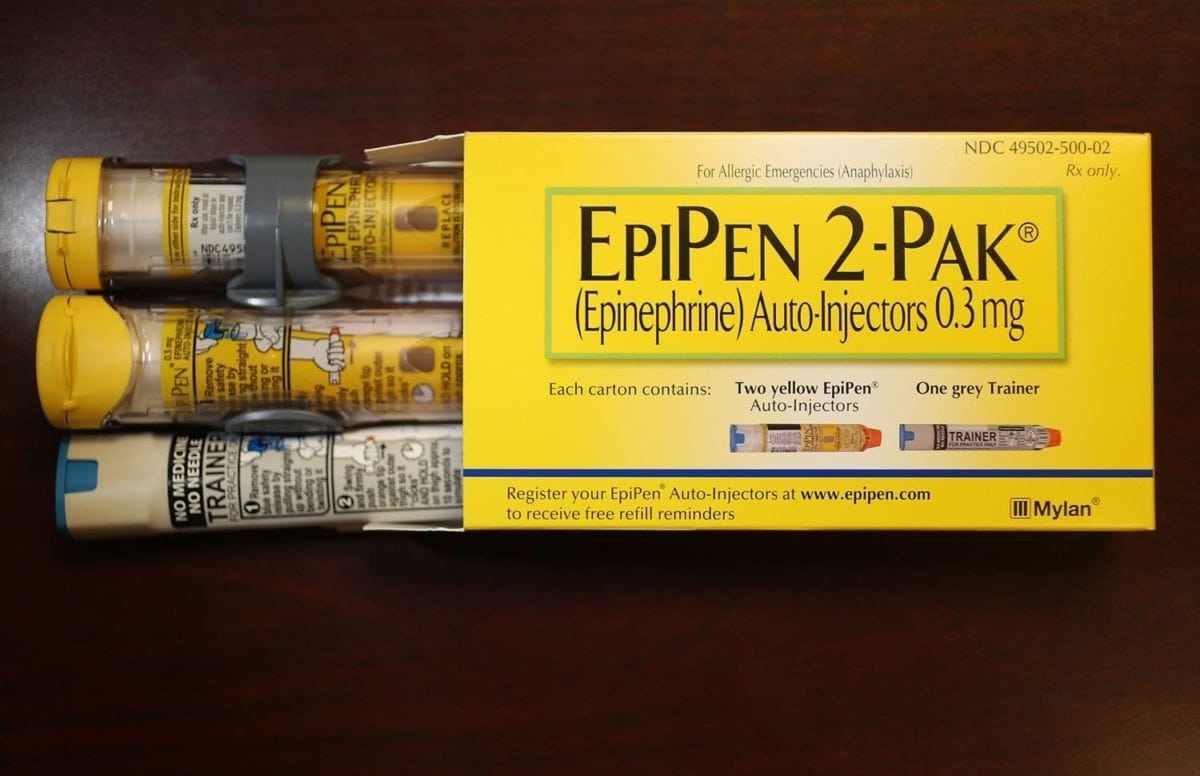
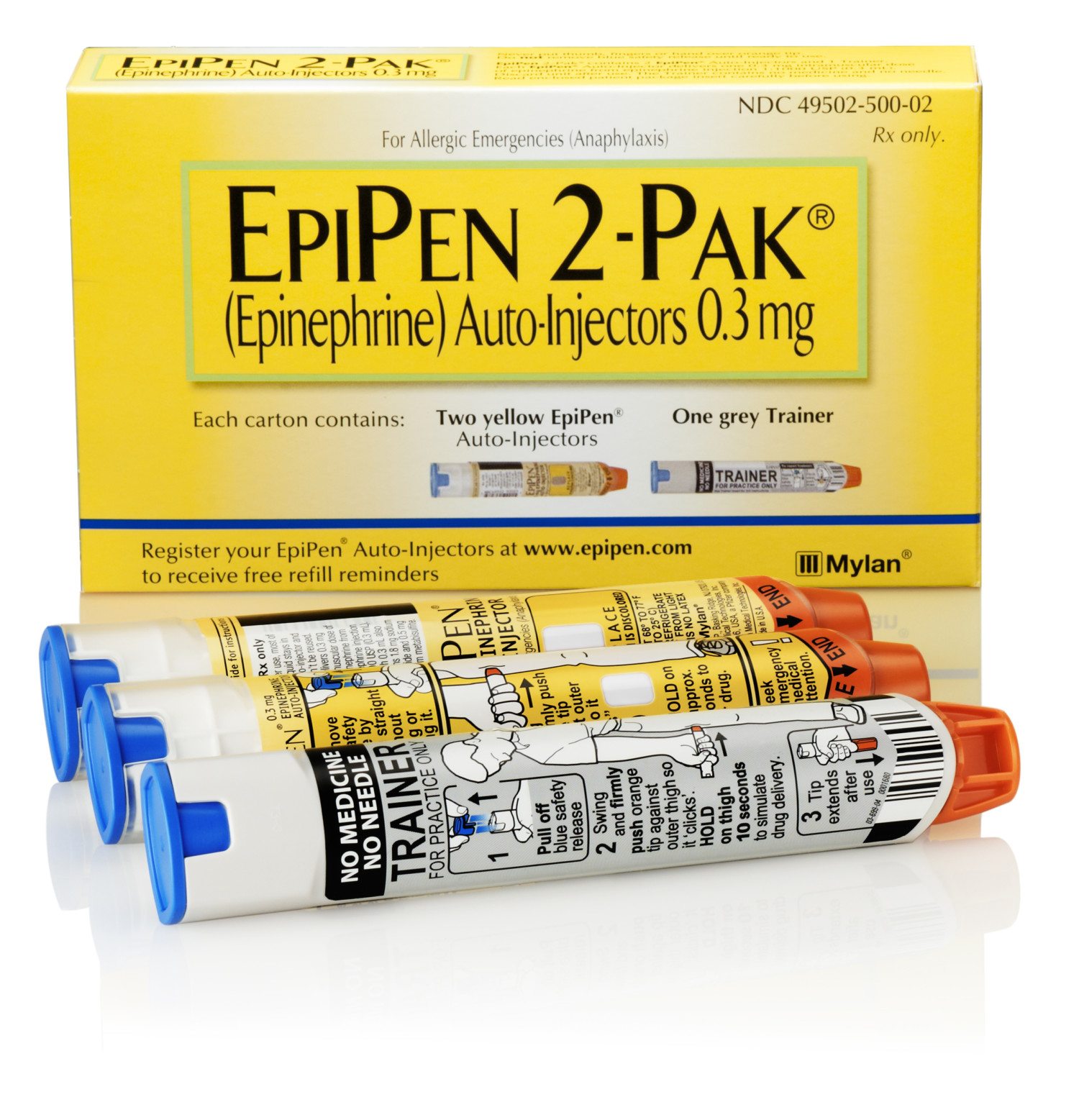
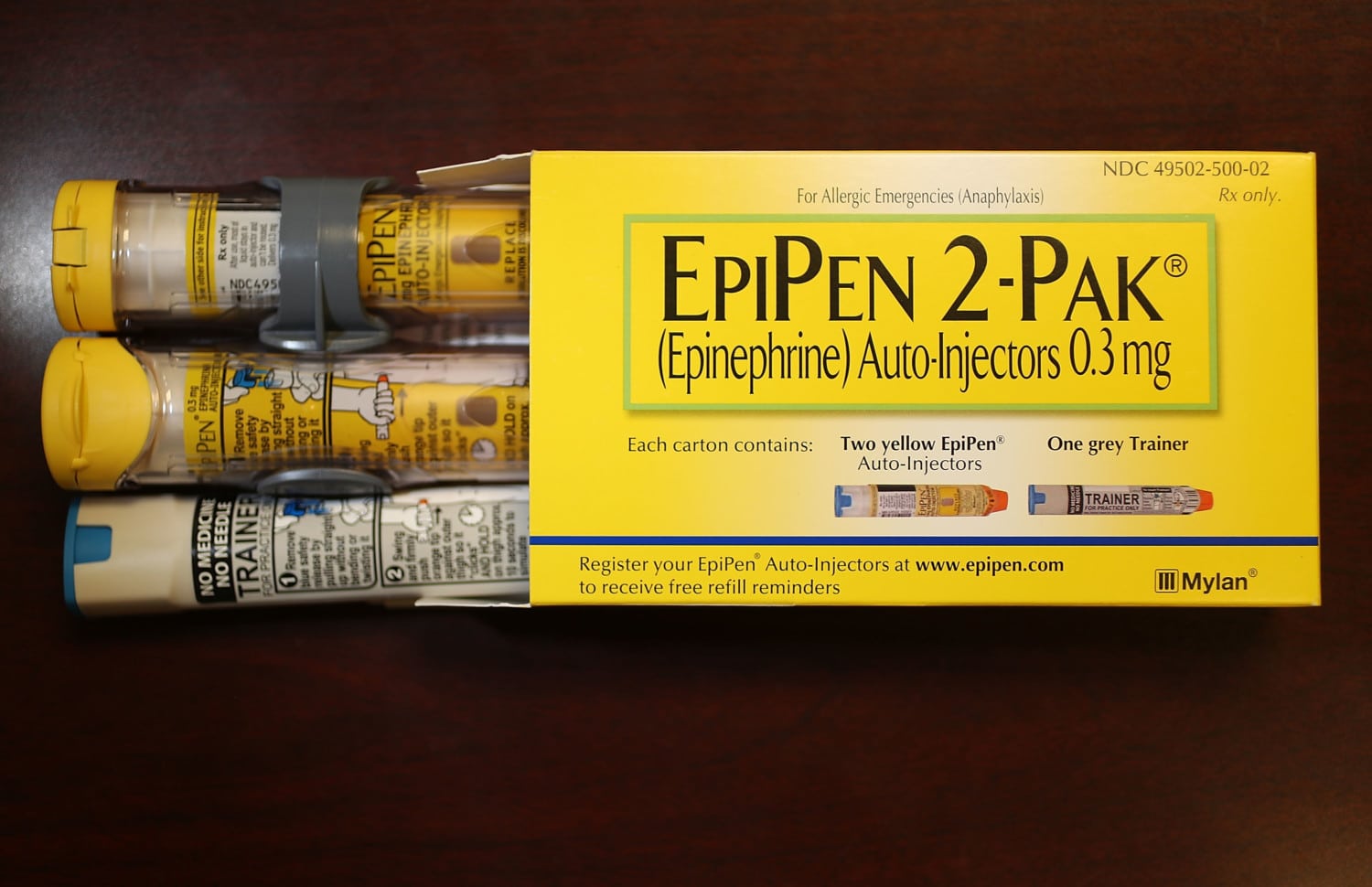
FDA Approves the First Generic EpiPen
Late last week, the Food and Drug Administration approved the first generic version of EpiPen to treat emergency and life-threatening anaphylaxis. The approval comes two years after pharmaceutical company Mylan increased the cost of EpiPens by more than 400 percent, leading to shortages of the drug and outrage amongst allergy-sufferers.
The generic version of EpiPen and EpiPen Jr. from Teva Pharmaceuticals USA received the green light from the FDA to be sold as the therapeutic equivalent to the brand-name auto-injector, meaning a pharmacy can automatically substitute it for prescriptions for EpiPen or EpiPen Jr.
“Today’s approval of the first generic version of the most widely prescribed epinephrine auto-injector in the U.S. is part of our longstanding commitment to advance access to lower cost, safe and effective generic alternatives once patents and other exclusivities no longer prevent approval,” FDA Commissioner Scott Gottlieb, M.D., said in a news release.
“This approval means patients living with severe allergies who require constant access to life-saving epinephrine should have a lower-cost option, as well as another approved product to help protect against potential drug shortages.”
A Necessary Move Amidst Rising Costs
While the availability of a generic version of the drug might seem like a no-brainer, allergy-sufferers have been fighting tremendous price increases — a two-pack of EpiPens went from about $57 in 2007 to $600 in 2016 — that present an often insurmountable roadblock to obtaining the drug. In May, EpiPens were added the FDA’s drug shortage list.
The price hike sparked outrage in users of the drug and allergy-awareness advocates alike, including actress Sarah Jessica Parker who had been a spokesperson for Mylan, but cut ties with them two years ago, once she learned of the price increases.
Parker said via Instagram, “I’m left disappointed, saddened and deeply concerned by Mylan’s actions.”
https://instagram.com/p/BJh35t2D3d8/?utm_source=ig_embed
Parker had been part of Mylan’s “Anaphylaxis for Reel” campaign, created to draw attention to life-threatening allergies like the type Parker’s 13-year-old son has to peanuts and hazelnuts.
A Long Road To Approval
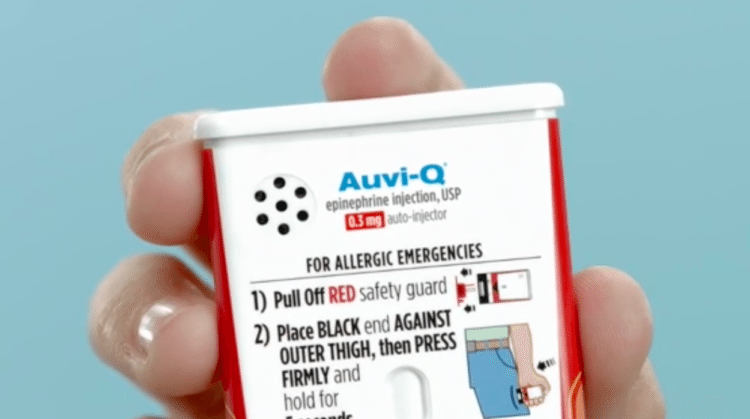
Amidst the criticism, Mylan began to offer its own generic version of the EpiPen for about half the cost, as did other companies, including Auvi-Q and Adrenaclick, an alternative epinephrine injector that could be obtained for as low as $142 from Sam’s Club and Walmart.
But this new option made by Teva is the only FDA-approved non-brand option.
“The path to developing generic drug-device combination products like this one is challenging,” Gottlieb said. “These products can be hard to copy, and therefore sometimes don’t face timely generic competition once patents and exclusivities are no longer a block to approval.”
While the news of an FDA-approved generic alternative to the EpiPen is welcome indeed, there is still no information on the price of the product or when it will become available. A statement from the company referred to a launch “in the coming months.”
A New Day For Allergy-Sufferers
The reaction to the approval was widespread, and included comments from lawmakers and EpiPen users. Senator Tina Smith wrote that the decision was “long overdue”:
A generic version of EpiPen has been approved—this was long overdue, and one of the many things we need to do to address the skyrocketing price of prescription drugs. I'll soon intro a new bill to ↓ Rx prices too. Price-gouging has to stop. https://t.co/sQcIhz05hY
— Senator Tina Smith (@SenTinaSmith) August 16, 2018
Although there is no information yet on an exact launch date for the generic product, some people lauded the timeliness of the decision as kids head back to school, including @_jacquie_lee who said it would ease a major concern for parents of kids with allergies to know that relief is on the horizon:
New FDA approval for EpiPen generic has good timing: kids are poised to start school and parents are concerned about the EpiPen shortage
— Jacquie Lee (@_jacquie_lee) August 16, 2018
Meanwhile, some EpiPen users described the scary reality they had faced prior to the approval, including Greg Sarafan, who mentioned his expired EpiPen and wrote, “Yay! It doesn’t cost hundreds of dollars for me not to die!”
I've been saving my EpiPen from a few years ago and I've been telling myself it's still good bc it's still in the box. Maybe now I will finally get a new one that isnt expired.
— Greg Sarafan esq (@GSarafan) August 16, 2018
The approval certainly serves as the best news allergy-sufferers have heard in a long time.